ToRCH is an acronym for a group of infections that can cause significant birth defects and even fetal death with “T” for Toxoplasma gondii, “R” for rubella virus, “C” for cytomegalovirus, and “H” for herpes simplex virus, and “O” for Other. Pregnant women infected with ToRCH have no obvious symptoms or mild symptoms. However, it can be vertically transmitted to the fetus, causing intrauterine infection, abortion, congenital malformation, premature delivery and perinatal death, etc. It can also cause multi-organ and multi-system function damage in newborns, and lead to different degrees of intellectual impairment in newborns.
- Toxoplasma gondii
Toxoplasma gondii is an obligate intracellular parasite. The domestic cat is its main host. Statistics show that the infection rate of toxoplasma during pregnancy is 20-30% in the United States and 50-80% in the United Kingdom. When infected with T. gondii during pregnancy, the brain and eyes are the main organs damaged in the fetus. Early pregnancy infection, cause abortion, stillbirth; During the second and third trimester of pregnancy, although most fetuses can continue to grow and develop in utero, they are prone to preterm birth or developmental defects after delivery.
Three secretory organelles are present in the cytoplasm of T. gondii: microneme, rhoptry, and dense granules, which are unique to parasites of the phylum Apicomplexa and are known to assist parasite entry and maintain intracellular host-parasite relationships. Microneme proteins (MICs) include transmembrane and soluble proteins expressing different adhesive domains. Upon secretion and redistribution on the parasite surface, transmembrane MICs are thought to connect external receptors to the submembranous acto–myosin motor that provides the power for parasite gliding and host cell invasion. Rhoptries (ROPs) are known to be involved in an active parasite’s penetration into the host cell associated with the biogenesis of specific intracellular compartment, parasitophorous vacuole in which the parasite multiplies intensively, avoiding intracellular killing. Dense granule proteins (GRAs) are identified as excretory/secretory antigens (ESP). Most GRA proteins are secreted into the parasitophorous vacuole (PV) and contribute to the maturation and structure integrity of the PV.
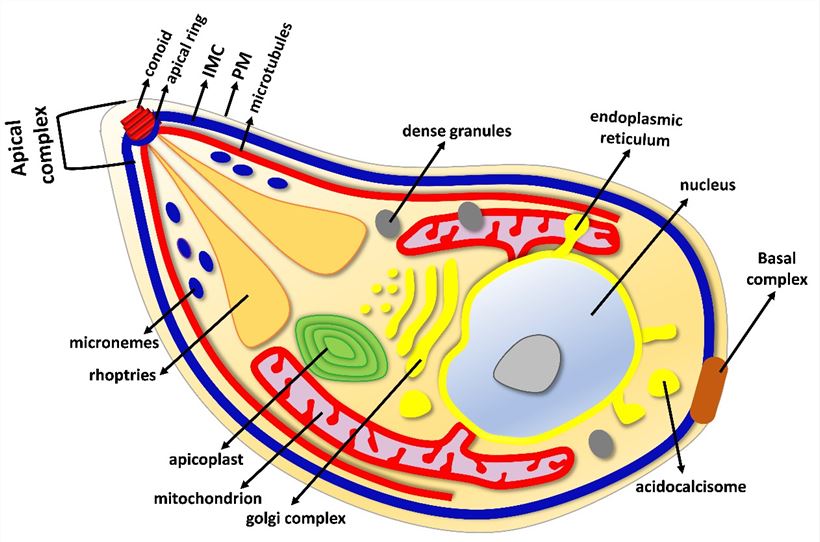
Fig 1. The major organelle structures of T. gondii tachyzoite (Günay-Esiyok, Özlem. 2019)
- Rubella virus (RuV)
Rubella virus is an RNA virus that causes rubella through the respiratory tract. Humans are the only hosts for the virus. Rubella virus can cause vasculitis, leading to ischemia of developing organs, resulting in fetal growth restriction. The organs most commonly affected by rubella virus in fetuses are the eyes, heart and nervous system, which are manifested as hearing disorders (60-75%), heart abnormalities (10-20%), eye defects (10-25%) and central nervous system abnormalities (10-25%), etc. Newborns infected with rubella virus often have a typical triple malformation, namely congenital heart disease, deafness, and cataracts.
Rubella virus particles have a diameter of about 60 nm. There is a nucleocapsid exhibiting cubic symmetry surrounded by a lipoprotein envelope with spiky projections consisting of two glycoproteins, E1 and E2. E1 carries the principal antigenic determinants and is responsible for receptor recognition and low-pH-triggered membrane fusion upon internalization through receptor-mediated endocytosis. E2 is topologically buried under E1 on the viral surface and plays a role in the cell surface expression of E1.
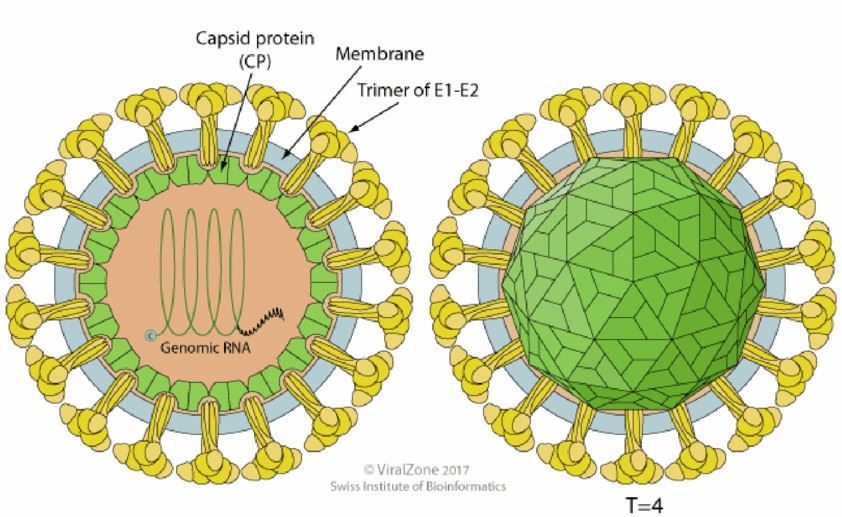
Fig 2. Schematic representation of the rubella virus structure (Pittet, Laure. 2018)
- Cytomegalovirus (CMV)
Cytomegalovirus, which belongs to the herpesvirus family, has a typical double-stranded DNA structure and is most common in intrauterine infections. The incidence of neonatal malformations due to primary cytomegalovirus infection during pregnancy is about 10%. Cytomegalovirus infection is harmful to fetuses and newborns, and early pregnancy infection is prone to spontaneous abortion, stillbirth, fetal growth restriction, etc.
The virion of cytomegalovirus is approximately 230 nm in diameter. It is composed of a nucleocapsid, surrounded by a less structured tegument layer, and bounded by a trilaminate membrane envelope. Its diameter is about 110 nm and it is isosahedral. Accommodating larger DNA in a similar diameter capsid may be achieved by eliminating the maturational protease (pUL80a) from the interior of cytomegalovirus capsids. The capsid is composed of four integral protein species (for human cytomegalovirus, pUL46, pUL80.5, pUL85, pUL104) that are organized into 162 capsomeres (150 hexamers plus 12 pentamers) and 320 triplexes located between the capsomeres.
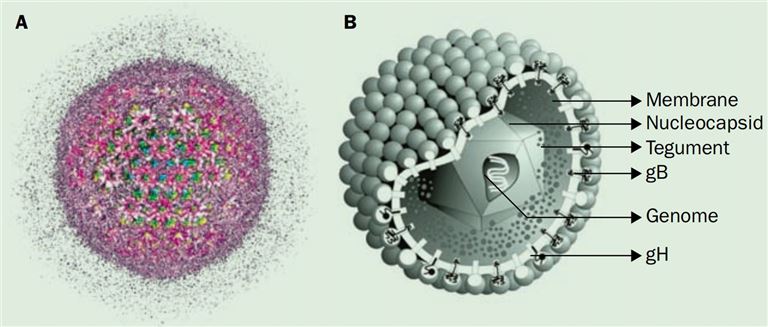
Fig 3. Schematic representation of the human cytomegalovirus structure (Gandhi, M. K., & Khanna, R. 2004)
- Herpes simplex virus (HSV)
HSV rarely presents with in utero infection but instead presents due to perinatal exposure. Therefore, clinical manifestations normally will present ten to twenty-one days after infection. There are three major manifestations: Skin-eye-mucous membranes (SEM), central nervous system (CNS), and disseminated disease. All will often present with fever in the neonatal period. The disseminated disease will present earliest after approximately one week of age.
HSV are composed of a large, double-stranded DNA core, an icosohedral capsid, an amorphous protein tegument, and an outer lipid bilayer envelope. The virus has a 152 kbp dsDNA encoding probably 84 proteins. The genomes of HSV are complex and contain two unique regions called the long unique region (UL) and the short unique region (US) that are flanked by inverted repeats. The tegument layer is an amorphous collection of at least 20 proteins which have significant roles in subverting the host antiviral response early after infection. The lipid envelope that surrounds the virus contains 11 viral encoded glycoproteins. It is responsible for attachment and penetration into the host cell, as well as inciting the host inflammatory response.
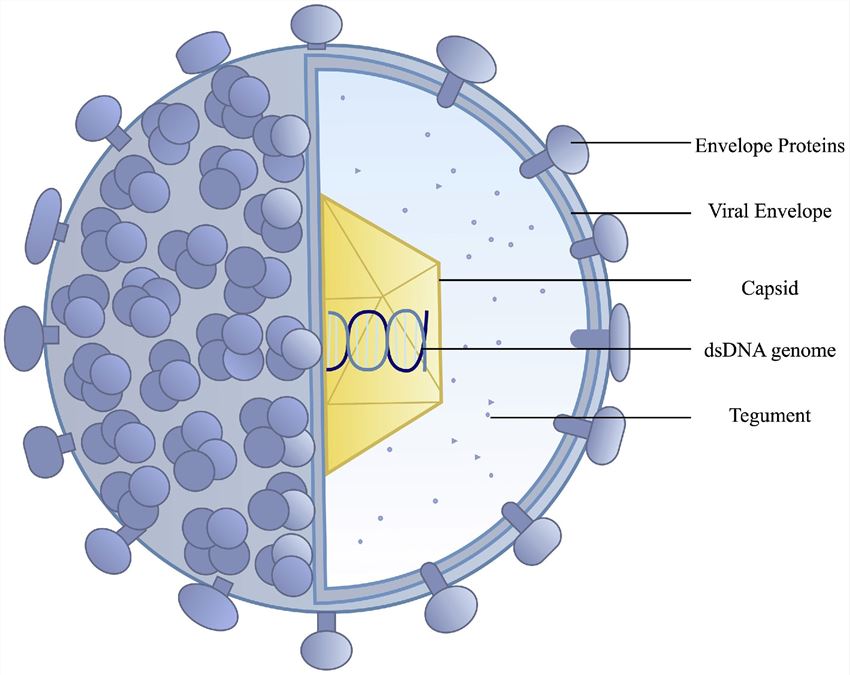
Fig 4. Schematic representation of the HSV structure (Gandhi, M. K., & Khanna, R. 2004)
Differential Diagnosis
As many of these illnesses have similar manifestations, they are often all considered as a possible diagnosis when a child presents with signs and symptoms suggestive of congenital infection. Therefore, in a child who presents small for gestational age with additional clinical findings such as a rash or heart murmur or ocular findings, all of the ToRCH complex pathogens should be considered. In addition to those mentioned, the viral pathogen Zika virus can cause significant disease in newborns. In particular, the virus causes significant CNS disease and can present with intracranial calcifications. Parvovirus B19 infection can also cause fetal hydrops fetalis and can present with profound bone marrow suppression.
Maternal factors such as preeclampsia, hypertension, smoking, drug and medication use, and anemia may contribute to growth problems. Additionally, many metabolic and genetic syndromes can present in a similar manner to a ToRCH infection. These may range from common issues like hypothyroidism to much more complex and rare genetic syndromes.
Laboratory Detection
ToRCH antibody serological assays are available for the detection of pathogen IgG and IgM antibodies for Toxoplasma gondii, rubella, CMV, and HSV, which can be used in a variety of assay formats including ELISA, rapid assays, and bead-based assays. Reactivity for IgM, but not IgG, usually indicates a current infection, while IgG without IgM suggests a past infection. However, for some pathogens, including toxoplasmosis and CMV, IgG can indicate a primary infection and the use of IgM is not considered reliable. Paired-serological tests are probably the most useful technique for analyzing the mother’s blood; a blood sample is taken during symptoms of the illness and the test is repeated four weeks later to determine any changes in the IgM/IgG antibody titers and avidities, which are used for diagnosis. In rapid IgM capture assays, which facilitate sensitive diagnosis of early-stage infections, an anti-IgM antibody fixed to a solid phase is used to capture pathogen IgM in patient samples. Immunofluorescent antibody assays (IFA) can also be used to definitively diagnose certain ToRCH agents and may be necessary in some cases to distinguish between strains, e.g., HSV-1 and HSV-2. Immunological and serological tests can provide information on the mother’s likelihood of infection; however, the pathogens may not cross to the fetus. To confirm congenital infection, molecular analysis of amniotic fluid is usually recommended.
References
- Chen, L., Liu, J., Shi, L., et al. (2019). Seasonal influence on TORCH infection and analysis of multi-positive samples with indirect immunofluorescence assay. Journal of Clinical Laboratory Analysis, e22828.
- Macedo-da-Silva, J., Marinho, C. R. F., Palmisano, G., et al. (2020). Lights and Shadows of TORCH Infection Proteomics. Genes, 11(8), 894.
- Günay-Esiyok, Özlem. (2019). Cyclic GMP signaling during the lytic cycle of Toxoplasma gondii.
- Pittet, Laure. (2018). Vaccination des sujets immunosupprimés.
- Gibson, W. (2008). Structure and Formation of the Cytomegalovirus Virion. Human Cytomegalovirus, 187–204.
- Gandhi, M. K., & Khanna, R. (2004). Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. The Lancet Infectious Diseases, 4(12), 725–738.
- Krishnan Rohini, Stuart Patrick M. (2021). Developments in Vaccination for Herpes Simplex Virus. Frontiers in Microbiology, 12.
